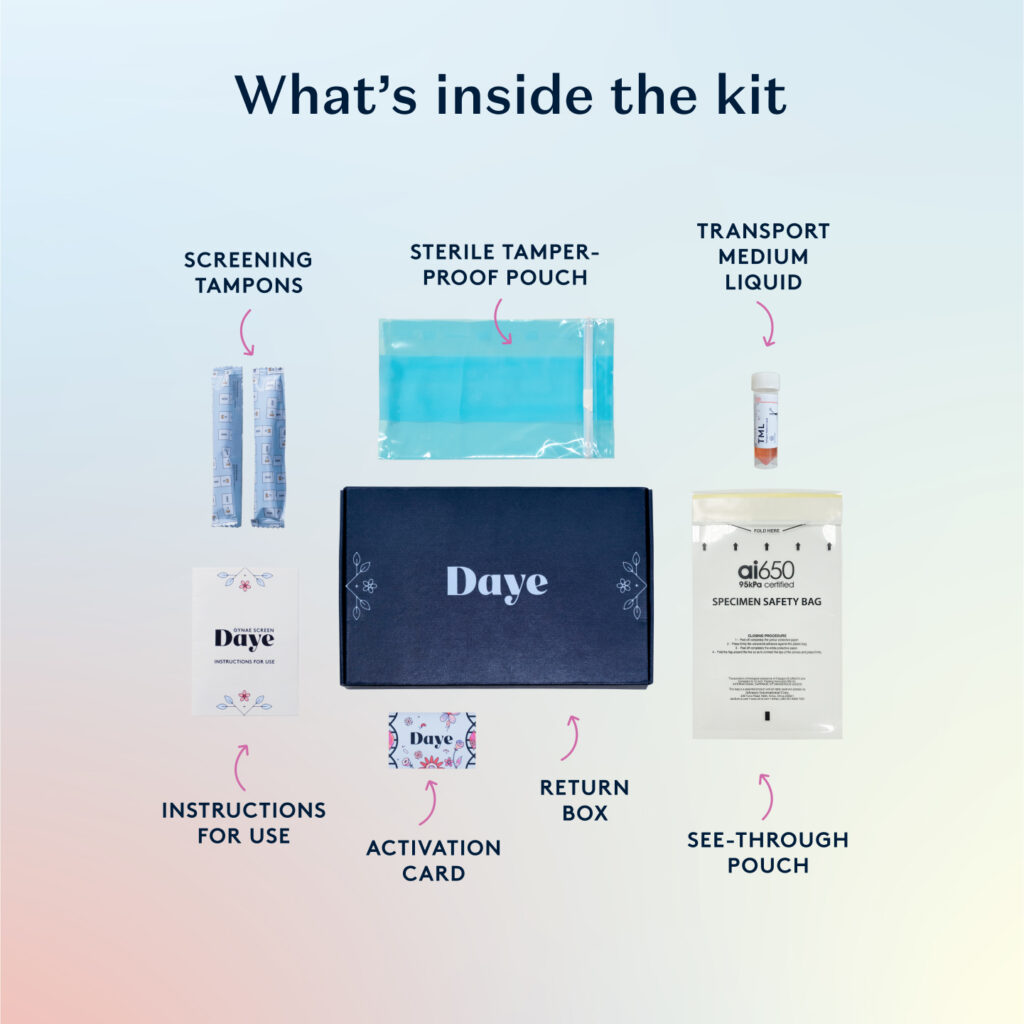
● Award-winning UK gynaecological health company Daye is launching an at-home HPV tampon-based testing kit
● Thepioneering kit enables women and AFAB individuals to test for 14 high-risk HPV infections
● 99.8%ofcervical cancer cases are caused by persistent high-risk HPV infections, while the virus is also linked to throat and oral cancer, as well as fertility problems
Award-winning gynaecological health startup Daye is today expanding its revolutionary at-home tampon-based screening kit to include HPV testing. The pioneering “Diagnostic Tampon” already utilises cutting-edge PCR testing technology to allow women and assigned female at birth (AFAB) individuals to screen their vaginal microbiome and test for STIs, such as chlamydia, gonorrhoea, trichomonas, mycoplasma and ureaplasma, from the comfort and convenience of their own home.
Daye is going one step further by introducing a screening kit for 14 high-risk HPV infections. Most strains of HPV do not present with any symptoms, which is why proactive screening is crucial. HPV infections are also incredibly common, eight in 10 people will become infected with HPV at least once in their lifetime[1], however, many still lack knowledge about the management of HPV and its potential complications. Nearly all cervical cancer cases (99.8%) are caused by persistent high-risk HPV infections[2], while the virus can also impact fertility and play a role in oral cancer[3].
Cervical cancer is one of the deadliest female cancers, and following the COVID pandemic, the NHS hasn’t been able to meet its cervical cancer screening target. Thanks to scientific breakthroughs, such as the HPV vaccine and the pap smear, cervical cancer rates have fallen by over a quarter since the early 1990s.
However, around 3,300 people still receive a cervical cancer diagnosis every year in the UK[4]. NHS England has set an ambitious target to eliminate cervical cancer by 2040[5], and Daye’s at-home HPV screen, which is part of the NHS Accelerator for Innovation, is set to support this ambitious goal.
Daye’s new HPV Diagnostic Tampon will allow people to collect a sample in the comfort of their own home and then discreetly send it to a lab that will return results within five to 10 working days. In addition to convenience, Daye’s Diagnostic Tampon increases testing accuracy, with only a 1% user error rate compared to 15% with traditional swabs, making it the simplest way to accurately screen for HPV at home. The patient samples will be analysed in a UKAS-accredited lab, following CQC-approved screening methodologies using a PCR-based, CE-marked diagnostic assay.
The Daye Diagnostic Kit is registered with the MHRA. Daye’s published, peer-reviewed research has found that patients and clinicians alike prefer the use of a tampon over a swab[6]. Used just like a normal tampon, Daye’s unique HPV Diagnostic Tampon collects more vaginal fluid than a standard swab and covers a larger surface area, making it more accurate than a swab and more comfortable than a speculum.
The Diagnostic Tampon is made with 100% organic, ethically sourced materials and comes with a sustainable applicator, which enables patients to reach their cervix without a speculum and feel confident in their ability to screen at home reliably. As part of the service, Daye will provide personalised aftercare, including prescription treatments and consultations with gynae health specialists. Daye will also help promote HPV vaccination programs, making HPV vaccines available as part of their aftercare service. If a patient tests positive for a high-risk HPV strain, they will be referred for a pap smear within Daye’s network of NHS specialists.
Valentina Milanova, Founder & CEO of Daye, comments: “Eliminating cervical cancer by 2040 requires a joined-up approach of vaccination, screening and testing, and we hope to encourage more women and AFAB individuals to proactively test for HPV. “By utilising the familiar tampon, this hassle-free HPV testing kit is extremely easy and comfortable to use, and we hope this will lead to more patients taking control over their long-term health and fertility.”
Notes to editors
1. Diane M Harper, Leslie R DeMars, HPV vaccines — A review of the first decade, Gynecologic Oncology, Volume 146, Issue 1, 2017, Pages 196-204, https://doi.org/10.1016/j.gyno.2017.04.004
2. NHS England- Cervical Cancer Screening– Don’t Ignore Your Invitation.
3. National Library of Medicine- Human papillomavirus infection and female infertility: a systematic review and meta-analysis.
4. Cancer Research UK- Just 12% of women know how HPV testing is used in cervical screening.
5. NHS England- NHS sets ambition to eliminate cervical cancer by 2040.
6. National Library of Medicine- Menstrual Tampons Are Reliable and Acceptable Tools to Self-Collect Vaginal Microbiome Samples. For more information, please contact Laura Tyther at lauratyther@gmail.com or on + 44 7492 311122.
About Daye Daye is a female-founded gynaecological health startup that is fast becoming the leading destination for menstrual pain management, vaginal health, and hormonal awareness. Founded in 2017, the company is on a mission to overcome historical gender biases and close the gender gap in medical research and innovation.
Daye aims to empower women and assigned female at birth (AFAB) individuals to take control of their gynaecological health by pioneering gynocentric research and creating revolutionary products and services for period pain, menstrual care and at-home testing for STIs, HPV and vaginal infections. Daye’s tampons are the only tampon s to have achieved ISO 13485 certification, meaning they reach the highest standards possible for medical devices. Daye was recognised by WIRED as one of London’s hottest startups in 2022 and 2019, and Daye’s founder & CEO, Valentina Milanova, was named on the 2023 Forbes 30Under30 Europe list.
For more information, please visit yourdaye.com and @yourdaye on Instagram, Facebook, TikTok and Twitter.